Given a choice between gaining some fat vs having metastatic cancer, what would you choose?
For a cancer patient, this is a straightforward answer, without a doubt everyone would agree to put on some fluffy mass. Just to be clear, I am not suggesting putting on fat protects against cancer but I am talking about a new therapeutic strategy that can potentially convert highly migratory cancer cells into harmless fat cells. In a publication in Cancer cell, Ronen et al. propose a novel drug combination that can convert invasive cancer cells into fat cells. For this, the researchers exploit cancer cell plasticity – acquired property that helps cancer cells migrate to distant organs.
What is cancer cell plasticity?
Cancer-related deaths occur due to the migration of cancer cells from their organ-of-origin to distant organs, leading to multi-organ failure. This process of spreading of cancer cells to other organs is termed metastasis. However, metastasis is not an efficient process and cells have to go through at least four stages to form clinically relevant metastasis. i) overcoming basement membrane barrier and invasion into the stroma ii) entering (intravasation) and surviving in the circulation iii) exiting the circulation (extravasation) and infiltrating the remote organ iv) colonizing the new organ to form tumors. During this multi-step process, cancer cells acquire cellular and phenotypic plasticity to adapt to different environments. This plasticity allows cells to change their identity or phenotype and make them more adaptable in diverse environments. For example, an epithelial cell can change into a mesenchymal cell and vice versa. Ronen et al. used this cellular plasticity against the cancer cells to turn them into harmless fat cells.
How to convert cancer cells into fat cells?
With the aim to exploit the plasticity of cancer cells to direct them towards adipogenesis (formation of fat cells), the researchers first tested well known adipogenesis inducing combination of insulin, dexamethasone and Rosiglitazone (potent PPARy agonist) along with bone morphogenetic protein-2 (BMP2) in epithelial (Py2T), mesenchymal (MTΔECad) and pre-adipocyte fibroblast cells (3T3-L1). They found that mesenchymal breast cancer cells readily turned into adipocytes as visualized by lipid droplets of Nile Red and increased expression of C/EBPalpha (regulator of adipogenesis) while epithelial cells fail to do so. Suggesting that only the cells undergoing epithelial to mesenchymal transition acquire the plasticity needed for trans-differentiation into adipocytes. On further investigation of these trans-differentiated cells, the researchers found that the combination of Rosiglitazone and BMP2 was able to induce functional adipogenesis in mesenchymal breast cancer cells supported by the expression of adipocyte-specific markers.
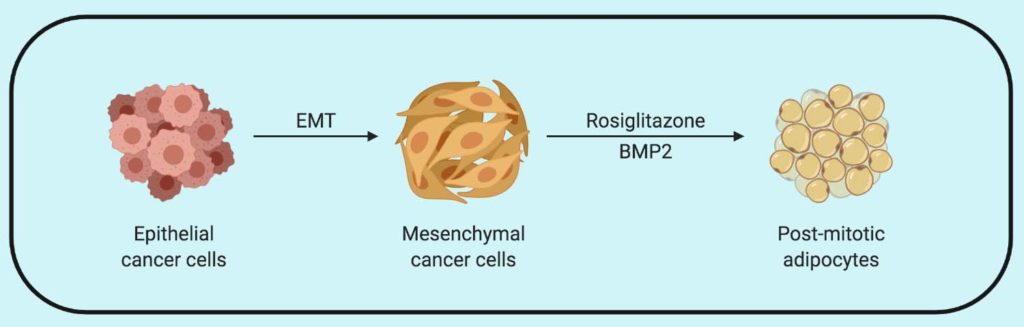
Furthermore, the differentiated cells demonstrate reorganization of cortical actin and didn’t revert back to the epithelial or mesenchymal state when the treatment was removed. The researchers then look at the changes in trans-differentiated cells using RNA-Seq data and found downregulation of oncogenes and cell-cycle related genes and upregulation of tumor suppressor genes, suggesting cancer cells were irreversibly differentiated into adipocytes with post-mitotic cell cycle arrest.
Dissecting the regulators of cancer cell adipogenesis
One of the underlying features for this trans-differentiation was that only the cells undergoing EMT, but not epithelial cancer cells, were able to undergo adipogenesis. A number of EMT and adipogenesis regulating transcription factors were upregulated in cells undergoing this transition. The researchers further looked into TGFβ pathway, which is an inducer of EMT but repressor of adipogenesis. As expected, the presence of TGFβ suppressed the adipogenesis and its inhibition promoted it. On further analysis, the researcher noticed activation of the non-canonical TGFβ pathway going through MEK/ERK signaling node as responsible for inhibition of adipogenesis. Indeed, inhibition of MEK signaling even in the presence of TGFβ was able to promote adipogenesis. Since, TGFβ induces EMT via the canonical pathway but suppresses adipogenesis via non-canonical MEK/ERK pathway, targeting MEK/ERK signaling downstream of TGFβ enables efficient adipogenesis of EMT -derived cancer cells.
Going In vivo
To test the significance of the drug combination in real life, the researchers used their drug combination with a high and low dose of MEK inhibitor PD98059 along with Rosiglitazone in animal models of invasive breast cancer. They realize that the higher dose of PD98059 was toxic so they had to terminate the experiment. However, they observed that lipid markers such as BODIPY were present in higher amounts in the drug combination-treated mice tumors compared to control. Notably, the adipocytes were present in the invasive front of the tumor, where the most invasive cancer cells with higher plasticity reside. The group then replaced PD98059 with FDA approved MEK inhibitor Trametinib and tested its efficacy in mice transplanted with metastatic breast cancer mouse models transplanted with ZsGreen expressing 4T1 cancer cells. Further analysis of the invasive front of the tumor revealed a reduction in the invasiveness of the tumor and increased in ZsGreen positive adipocytes in combination-treated tumors.
In a more invasive breast cancer model MDA-MB-231 cell lines (known to metastasize to the lungs) treatment with Rosiglitazone and Trametinib combination didn’t have a significant impact in primary tumor growth compared to Trametinib alone. However, the combination successfully repressed tumor invasion and metastasis to the lung.
Validation in a preclinical model
To further, assess the adipogenic potential of Rosiglitazone and Trametinib combination in a clinically relevant setting, the researchers used the PDX model of human breast cancer carrying PI3KCA, BRCA1, and TP53 mutations. Mice were implanted with patient tumor material and the primary tumor was allowed to grow for 4 weeks followed by drug treatment for the next 4 weeks. Primary tumors were then surgically removed and the mice were kept under therapy up to 4 months post-transplantation. The primary tumors from combination treatment demonstrated a higher number of HLA-A and perilipin (tumors cells with adipocyte marker) compared to control and individual treatments validating the adipogenic potential of the combination treatment. Furthermore, the combination treatment significantly suppressed lung metastasis formation and outgrowth compared to the controls.
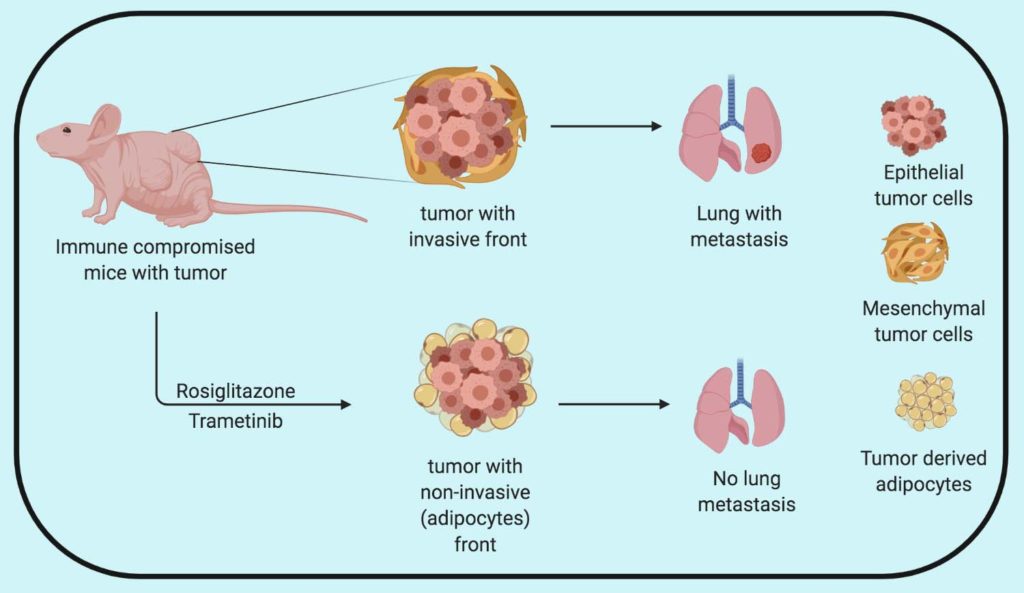
Overall, Ishay-Ronen et al. demonstrate that the combination of Rosiglitazone and Trametinib can be used to exploit the cancer cell plasticity during epithelial to mesenchymal transition (EMT) to induce cancer cell trans-differentiation into adipocytes. Although the combination therapy reduces the metastasis to lungs, going into the clinic as a metastasis inhibitor is a bit tricky as patients might already have metastatic tumors. The question that comes to my mind is how can we make use of this cool approach to treat cancer?
By Shishir Pant
Reference
Ishay-Ronen, D., et al. (2019). “Gain Fat-Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis.” Cancer Cell 35(1): 17-32 e16.

It is indeed a cool approach. A case can be made for slowing down the severity of the disease or increased standard of living for the patients at the current stage.
It is a cheeky approach 🙂 but something that has hindered drugs targeting metastasis for a long time is the fact that most of the patients might already have metastasis, by the time they are diagnosed. However, in my opinion, this combination can be very useful in preventing early breast cancer development especially in individuals with rigid breasts.