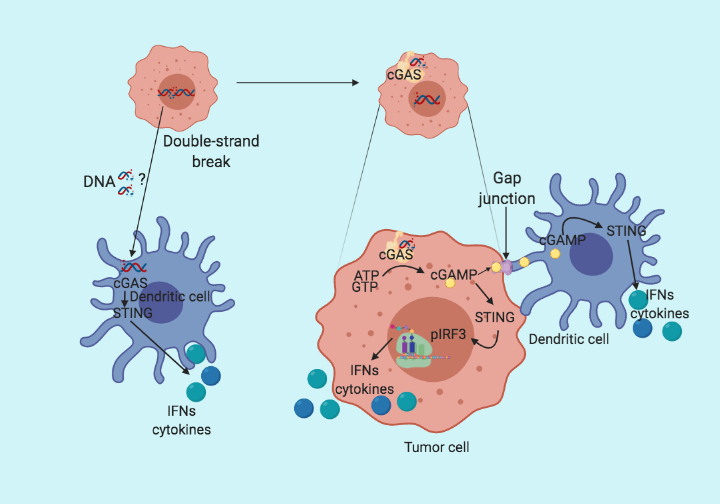
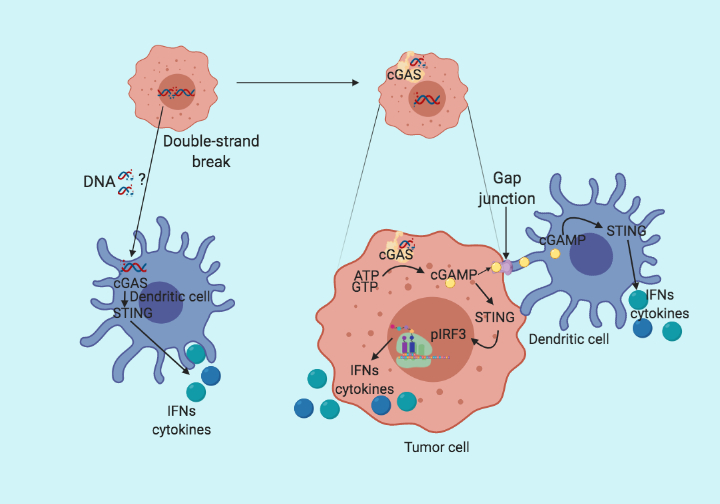
Following up on the previous blog (STING the Cancer). Here, I summarize a recent paper from Linda et al. published in the journal Cell Reports, which fills up the missing links in STING pathway activation. The conventional wisdom in STING pathway is that the cytoplasmic DNA is bound by cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS), which initiates the production of cGAMP. Subsequently, cGAMP binds to STING which then phosphorylate and activates interferon regulatory factor 3 (IRF3) and starts the production of type I interferons. All this happens in the same cells. However, in their recent paper Linda et al. claim that the cancer intrinsic cGAS expression but not STING is the determining factor for tumor immunogenicity. They further provide evidence that cGAMP produced by cancer cells is transferred via gap junctions to adjacent dendritic and macrophage cells, where the STING pathway is activated and leads to the recruitment of T cells.
Why do we care whether STING is activated in cancer cells or monocytes?
Due to the prevalence of a high concentration of cytoplasmic DNA in tumor cells, cancer cells mutate/inactivate components in STING pathway to suppress their immunogenicity. This is consistent with the observation that the inactivation of cGAs/STING pathway correlates with poor prognosis in cancer patients. So the mechanism of how the STING pathway gets activated is important if we want to therapeutically intervene in the pathway to turn non-immunogenic tumors into immunogenic.
cGAS expression in cancer cells is essential for dendritic cells mediated type I interferon production
To test whether cGAS expression is necessary for tumor immunogenicity, the group made cGAS and STING knockout cell lines using the CRISPR/Cas9 system. When co-cultured with bone marrow-derived dendritic cells (BMDC), cGAS mutant cells failed to induce INFy production in dendritic cells compared to control and STING mutant. Suggesting that cGAS but not STING is essential for dendritic cells mediated type I IFN production. On the other hand, the deletion of STING in BMDC abolished the type I IFN production. Implying that cGAS expression in cancer cells and STING expression in BMDC are critical for type I IFN production by immune cells. Based on the previous publications reporting the role of gap junction in cGAMP transfer, the group further generates connexin-43 deficient cancer cells. On co-cultured with BMDC, connexin-43 deficient cancer cells fail to upregulate type I IFN as compared to the control cell line. Suggesting cGAS in cancer cells sense the cytoplasmic DNA and produce cGAMP which is then transferred to the dendritic cells via gap junctions and activates STING in dendritic cells to induce type I IFN production.
cGAS deficiency in cancer cells makes tumors non-immunogenic
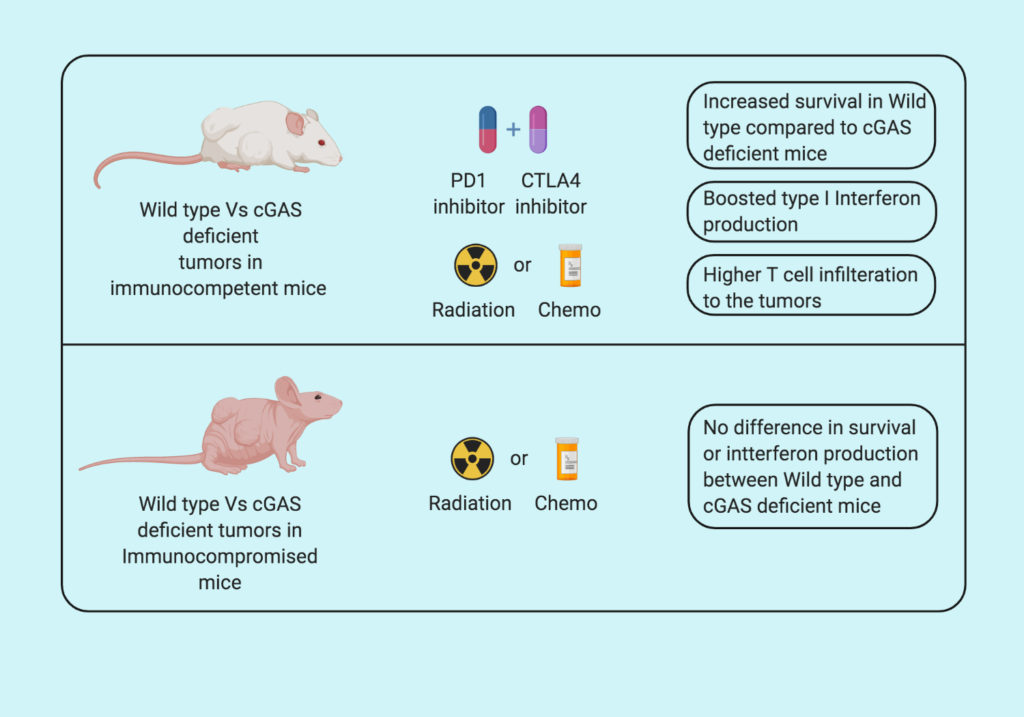
To further validate their findings in vivo Linda et al. injected the wild type (normal) CT26 cells, cGAS deficient CT26 cells, and STING deficient CT26 cells subcutaneously in BALB/c mice. They found increased tumor growth in cGAS deficient tumors compared to normal and STING deficient tumors. To further demonstrate that the tumor growth difference observed is due to lack of tumor immunogenicity, the group repeats the same experiment in immunocompromised NSG mice (NOD SCID gamma mice; lacking T, B, and NK cells). In immunocompromised mice all three tumors demonstrated similar growth and survival, suggesting that increased growth in cGAS deficient tumors is due to immune deficiency. On further analysis of the tumors, the group found decreased in cytotoxic T cells, IFNy and other immunogenic markers in cGAS deficient tumors but not in STING deficient tumors.
Thus providing further evidence that cancer cell-intrinsic cGAS promotes T cells infiltration and suppresses tumor growth.
Type I interferon producers in vivo
To test which cell population is responsible for increased type I IFN production in vivo, Linda et al. analyzed the tumor samples from cGAs deficient and control tumors using PrimeFlow. The found that tumor-associated macrophages and dendritic cells produced higher amounts of type I IFN in control tumors compared to cGAS deficient tumors. Further suggesting cGAs deficiency in tumor cells can downregulate IFN production via immune cells.
Moving to the Therapeutic Significance
Genotoxic agents such as chemotherapy and radiotherapy are known to induce DNA damage in cancer cells. Based on this observation, Linda et al. treated control and cGAS deficient mice with radio- and chemotherapy and followed the tumor progression. They found that both genotoxic agents increase the cytoplasmic DNA, increasing the infiltration of CD3+ and CD8+ T cells and lead to increased survival in control tumors compared to cGAS deficient tumors. Suggesting that cGAS is essential for sensing the DNA damage in tumor cells.
Furthermore, the group treated the cGAS deficient mice with the combination of two immune checkpoint inhibitors (anti-PD1 plus anti-CTLA4) and found that cGAS deficiency in tumors makes them less vulnerable to immunotherapy. Supporting the role of cGAS in increasing tumor immunogenicity.
The group then analyze the human tumor samples from colorectal cancer patients and find a good correlation between cGAS expression and the presence of infiltrating T cells. Suggesting, the presence of cGAS as a predictive marker for T cell infiltration or hot tumor.
To summarize, Linda et al. demonstrate that cGAS expression in tumor cells is important for the activation of STING pathway and type I interferon production in macrophages and dendritic cells. They further show that cGAMP produced in tumor cells can be transferred to the neighboring dendritic cells via gap junctions. Furthermore, cancer cell-intrinsic cGAS is essential for immunogenic tumor suppression and can potentiate responsiveness to immunotherapy.
By Shishir Pant
Reference
Linda et al. (2019) “Cancer-Cell-Intrinsic cGAS Expression Mediates Tumor Immunogenicity.” Cell Reports