CD4+ Regulatory T (Treg) cells suppress excessive immune responses thus maintain sanity in the host immune system. Cancer cells take advantage of the immune-suppressive features of CD4+ Tregs to dampen antitumor immune responses. Though the immunosuppressive role of Tregs is critical in malignant transformations, what’s not known is the mechanism of how CD4+ Tregs achieve their immunosuppressive capacity. Alvisi et al. report that the expression of transcription factor IRF4 by tumor-infiltrating CD4+ Tregs is a key determinant of their immunosuppressive capacity.
IRF4 expression defines intratumoral Treg heterogeneity
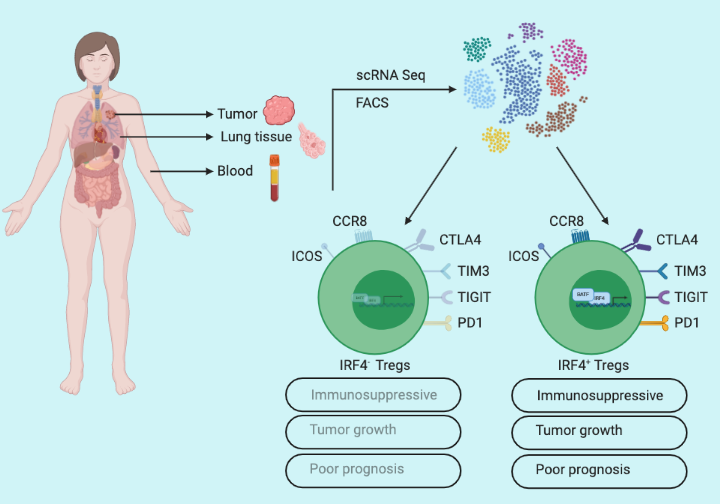
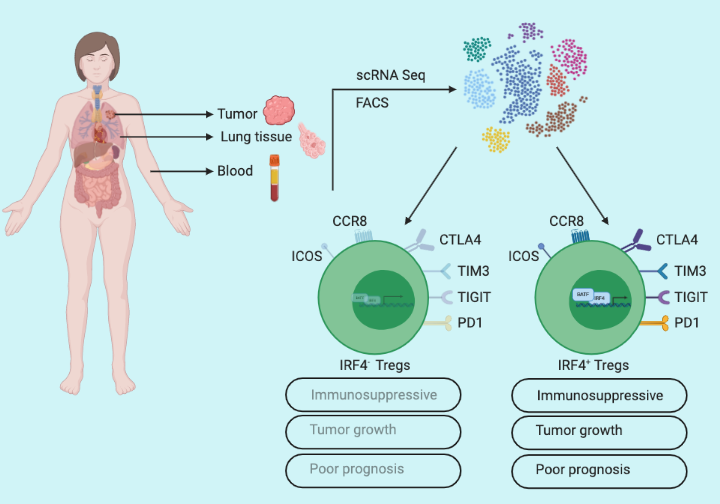
To understand CD4+ T cell phenotypes at the tumor site in non-small cell lung cancer (NSCLC) patients, Alvisi et al. used a single-cell approach to profile samples from three body sites: 53 lung tumors, paired-adjacent cancer-free lung tissue (n=45), and peripheral blood samples (n=22) of treatment naïve patients. Visualization of the transcriptomic data revealed a distinct single-cell profile, with enrichment of IRF4 expressing CD4+ Tregs in tumors compared to adjacent lung and blood samples. To further delineate Treg heterogeneity at the tumor site, Alvisi et al. performed flow cytometry analysis and identified approximately 40% of the tumor-infiltrating CD4+ Tregs expressed IRF4 (IRF4+) while remaining were negative (IRF4-). And they also noticed increased expression of Treg activation markers such as PD1, TIGIT, and TIM3 receptors in IRF4+ Tregs compared to IRF4– Tregs, suggesting a distinct function of IRF4 expressing CD4+ Tregs in tumors.
IRF4 expression vs Tregs suppressive potential in vivo
To gain a detailed insight into the functionality of IRF4in Tregs, Alvisi et al. performed bulk RNA-seq of IRF4+ and IRF4– Tregs. The analysis provided a list of 2674 differentially expressed genes in IRF4+ Tregs including a number of upregulated genes associated with effector Treg function. The transcriptional profiling indicated higher intratumoral activity of IRF4+ Tregs compared to IRF4– Tregs. To further validate, Alvisi et al designed an in vitro co-culture experiment and tested the capacity of IRF4+ and IRF4– Tregs to suppress the proliferation of conventional CD4+ T-cells (Tconv). Evidently, at a 1:1 Tconv/Treg ratio both subsets were effective, but at a 2:1 ratio, only IRF4+ Tregs showed effective suppressive capacity. To decipher in vivo functional significance of IRF4 in tumor-infiltrating Tregs, Alvisi et al. used the MC38 tumor-bearing mouse model, and conditional deletion of IRF4 in Tregs significantly delayed MC38 tumor growth illustrating antitumor immune-suppressive behavior of IRF4+ Tregs.
IRF4+ Tregs as a prognostic marker for NSCLC
Alvisi et al. characterized the T-cell phenotypic landscape in NSCLC patients utilizing single-cell profiling from tumors (n=45), paired-adjacent cancer-free lung tissue (n=23), and peripheral blood samples (n=23) of treatment naïve patients and identified 14 different CD4+ and 15 different CD8+ T-cell clusters. Further analysis revealed that IRF4+ Tregs positively correlated with CD4+ Tregs expressing PD1, TIGIT, and TIM3 (a marker for T-cell exhaustion), and negatively correlated with cytotoxic CD8+ T-cells. Finally, to test the significance of IRF4+ Tregs in cancer patient prognosis, they performed tumor glycolysis and aggressiveness analysis utilizing the maximum standardized value of fluorodeoxyglucose uptake (SUVmax) on a cohort with a subset of NSCLC patients. Importantly, SUVmaxhi patients harbored increased frequencies of IRF4+ Tregs while SUVmaxlo patients constituted increased frequencies of cytotoxic CD8+ T-cells. Furthermore, IRF4+ Tregs were enriched in patients with advanced pathological stages, and high Tregs/CD8+ T-cell ratio correlated with early tumor relapse.
In summary, Alvisi et al demonstrate IRF4 as a key transcriptional regulator of the immunosuppressive capacity of effector Tregs in the tumor microenvironment. They provide evidence that IRF4 expression dictates the capacity of effector Tregs to suppress the proliferation of conventional CD4+ T-cells and using xenograft mouse models further demonstrated the functional significance of IRF4 in tumor growth. They also evaluated the prognostic ability of IRF4+ Tregs in multiple cancer types including lung, melanoma, and hepatocellular carcinoma.
By Sushil Tripathi
Reference
Alvisi, G., et al. (2020). “IRF4 instructs effector Treg differentiation and immune suppression in human cancer.” J Clin Invest