Cells have evolved with an inbuilt self-destruction switch called apoptosis. The ability of cells to commit suicide helps us stay disease-free by removing damaged and worn-out cells. Cancer researchers are particularly interested in understanding this phenomenon in order to use it against cancer cells. In December 2018, 3 research articles were published in the same issue of journal Cancer Discovery and an article was published in Nature communications, all trying to induce apoptosis in cancer cells by targeting MCL-1 protein. Reinvigorating the interest in this class of inhibitors for cancer therapy.
So, let’s refresh our memory on apoptotic pathway
Apoptotic proteins
Apoptosis is a very precisely synchronized process which is regulated by a balance between pro-survival and pro-apoptotic proteins. The pro-survival protein, also known as BCL-2 family proteins (BCL-2, BCL-XL, BCL-W, MCL-1) are responsible for cell survival whereas pro-apoptotic BAX/BAK like proteins are responsible for

How is apoptosis regulated ?
For the pro-apoptotic protein to trigger cell death, BAX and BAK must oligomerize (form a complex) to form holes in the outer membrane of mitochondria which causes cell death. In healthy cells, pro-survival (anti-apoptotic) BCL-2 proteins bind with either BAX or BAK and prevent them from oligomerization and hence permeabilization of mitochondria. The BH3-only proteins, which are produced in response to various stress binds to BCL-2 family proteins. This releases BAX and BAK from BCL-2 family proteins, which then trigger apoptosis.

What happens in cancer ?

Several cancer types evade cell death by upregulating pro-survival BCL-2 family proteins like BCL-2, MCL-1, BCL-XL etc. Identification of different BCL-2 family proteins and their interactions with pro-apoptotic and BH3-only proteins has led to the development of BH3-mimetic drugs. These drugs bind to BCL-2 family protein like BH3-only proteins and activate BAX and BAK to trigger apoptosis. Despite decades of efforts, only BCL-2 inhibitor Venetoclax developed by AbbVie Inc. has been clinically approved. This highlights the complexity in developing selective inhibitors for this class of proteins.
So, what is the latest development in the field?
In the recent article published in nature communications, Tron A. E., et al. used structure-based drug design to develop a macrocyclic inhibitor (AZD5991) targeting MCL-1. Now in Phase I clinical trial. AZD5991 demonstrate high affinity towards MCL-1 and shows selectivity against other BCL-2 family proteins.

To address the selectivity issue
Tron A. E., et al. stably express BCL-2 family proteins in lymphoma cells and treat them with AZD5991. The drug-induced apoptosis in lymphoma cells stably expressing MCL-1 protein, which was abrogated when other members of BCL-2 family proteins such as BCL-2, BCL-xl, BCL-w, and BFL-1/A1 were expressed in the same cells. Demonstrating the selectivity against other BCL-2 family proteins, which has been a major problem in clinical trials for BH3 mimetics.
To determine the mechanism of AZD5991 triggered cell death
Tron A. E., et al. immunoprecipitated MCL-1 and showed its interaction with BAK. AZD5991 triggered the dissociation of MCL-1 and BAK complex and led to activation of BAK-dependent mitochondrial apoptotic pathways which was rescued in the absence of BAK.
To demonstrate the efficacy in multiple myeloma
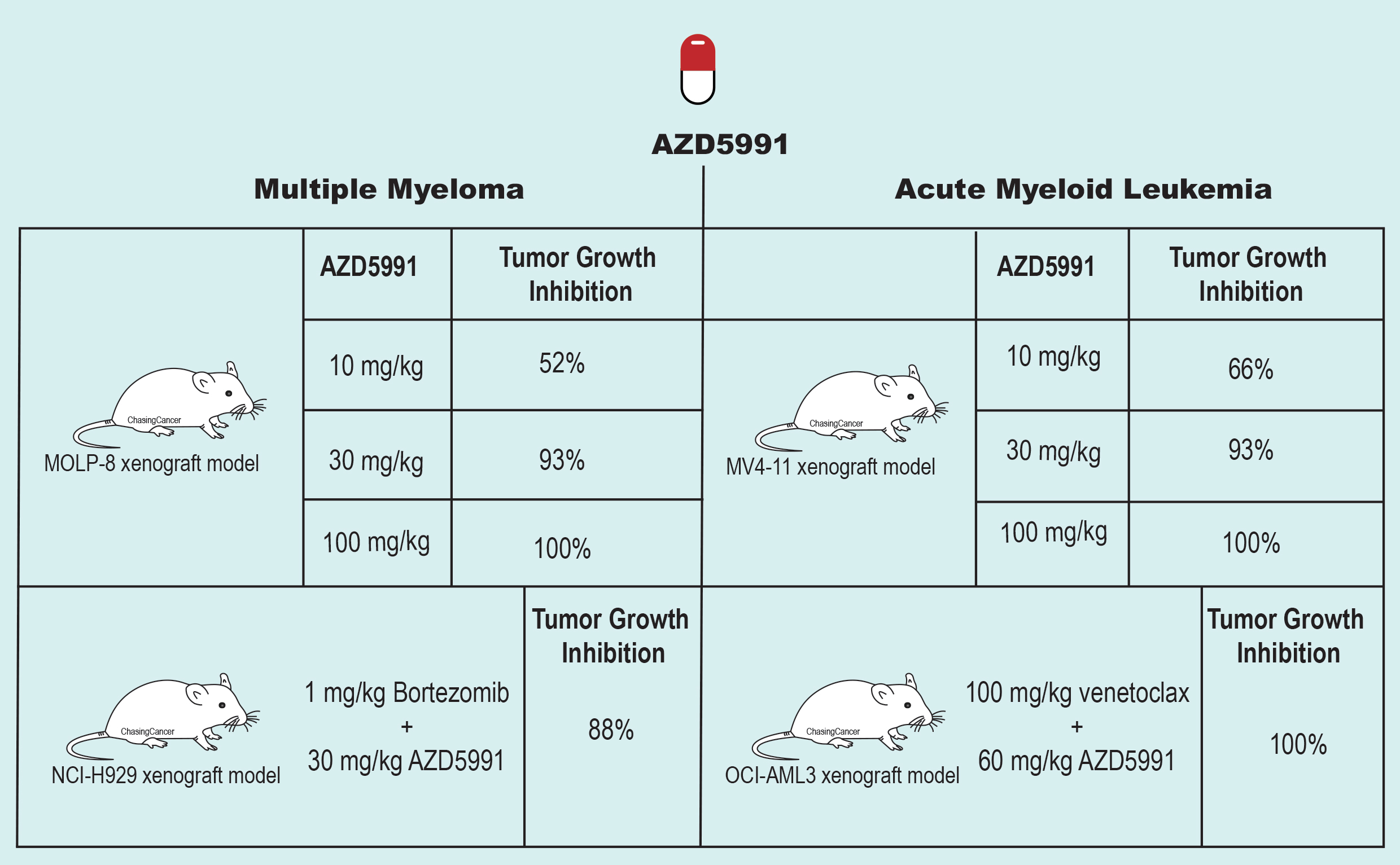
The team further validated the efficacy of AZD5991 in xenograft mouse models of multiple myeloma using MOLP-8 cell line. The single dose of AZD5991 at 60 mg/kg was able to give 99% tumor regression and at 100 mg/kg dose the tumor regression was 100%. They also treated primary cells from bone marrows of multiple myeloma patients with AZD5991 and observed 71% of 48 MM patients samples displayed EC50 value under 100 nM. Based on their observation that MCL-1 protein levels go up after the treatment with bortezomib (proteasome inhibitor) in NCI-H929 cells in vitro, the group further demonstrate that bortezomib sensitizes MM cells to MCL-1 inhibitor. In their in vivo experiment with NCI-H929 model grown subcutaneously, a sub-efficacious dose of AZD5991 (30 mg/kg) was able to induce 88% tumor regression in combination with bortezomib.
Efficacy in acute myeloid leukemia
Tron A. E., et al. further tested their compound in acute myeloid leukemia (AML) cells and xenograft mouse model using MV4-11 cell line. Achieving similar results as with the multiple myeloma xenograft; complete tumor regression with a single dose of 100 mg/kg AZD5991.
The group then screened panel of 11 AML cell lines with AZD5991 and
Overall, Tron A. E., et al. have developed a potent and selective inhibitor against MCL-1 which is now in phase I clinical trial. The inhibitor is able to induce tumor regression in xenograft mouse models of MM and AML. The group further show the potential for combining MCL-1 inhibitor with bortezomib and
However, the toxicity was assessed in mouse and rat, both of which have low binding affinity for human MCL1 inhibitor. Furthermore, MCL1 has
by Shishir Pant
Reference
Tron, A. E., et al. (2018). “Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia.” Nat Commun 9(1): 5341.