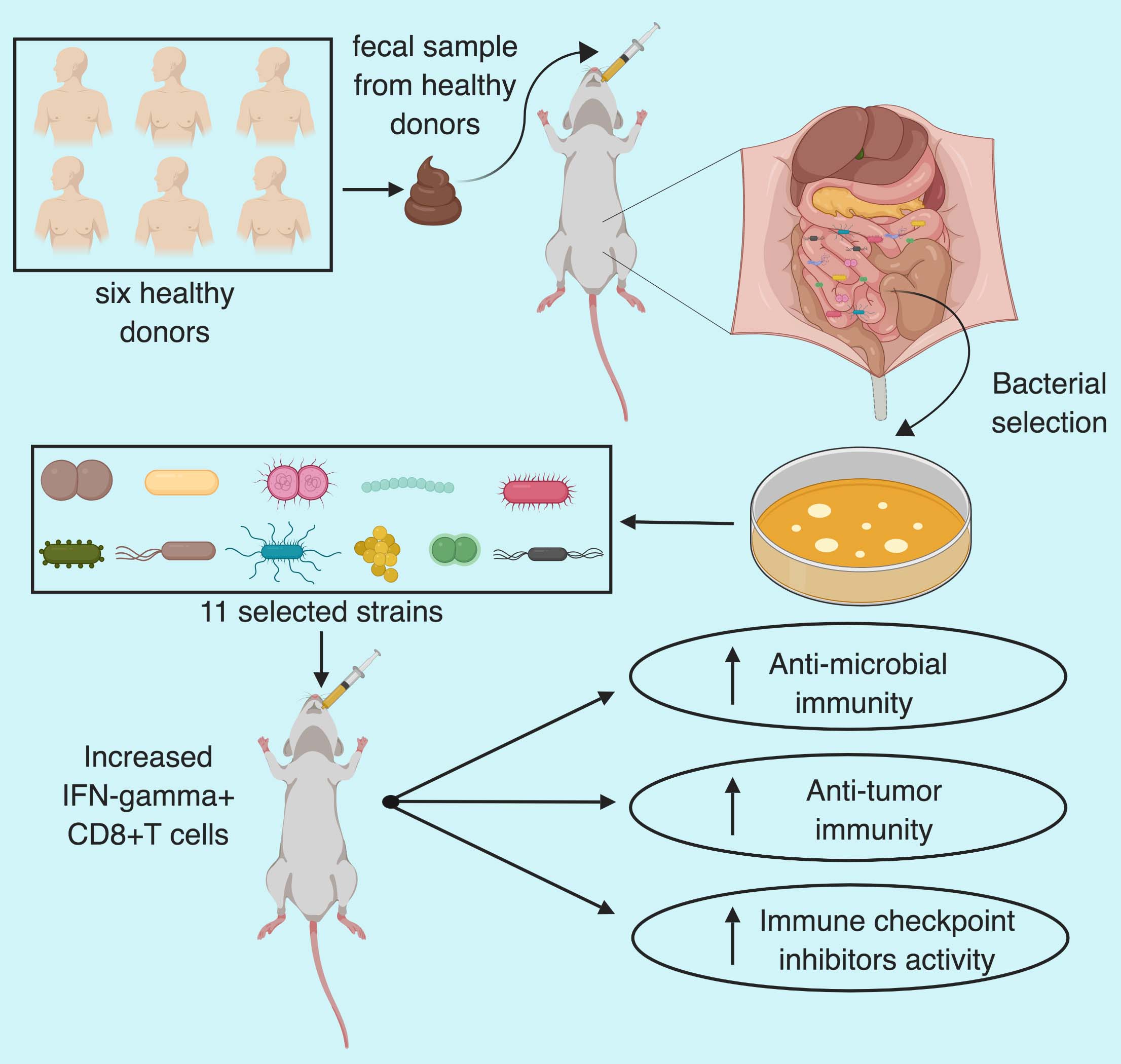
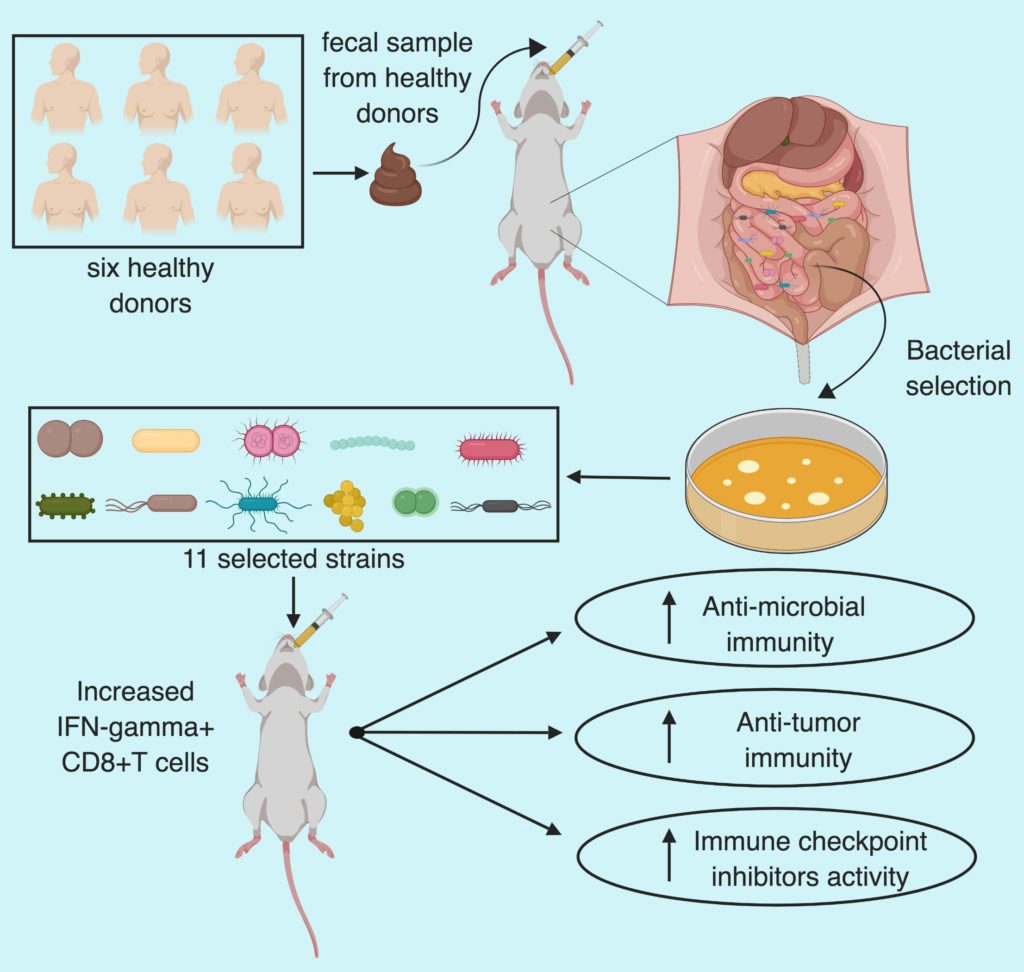
Immunotherapy has changed the landscape of cancer treatment with complete tumor remission in a subset of patients. While immense efforts are being made to increase the effectiveness of such treatment across a large population by combining them with other immune and targeted therapies. Attempts to decipher the variables which might influence the response to immunotherapy has led researchers to an unexpected field of gut microbiota. There is increasing evidence that the gut microbiota can influence the outcome of anticancer therapies such as immune checkpoint inhibitors targeting PD1 or CTLA4. However, one of the hurdles in developing microbiota-based therapeutics to aid cancer immunotherapy is the lack of known microbial species. In a recent article published in Nature, Tanoue and Morita et al. have isolated 11 bacterial strains, from healthy human microbiota, which induce IFNg+CD8+T cells and increase the efficacy of immune checkpoint inhibitors.
So, let’s recap what is an immune checkpoint inhibitor?
The immune system protects us from foreign microorganisms, removes toxins and destroys tumor cells without destroying our own organs. To do all these, it relies on a mechanism to discriminate between self and foreign, called immune checkpoints. Immune checkpoints are molecules expressed on the surface of the cells that signal to the immune system not to kill the cell. Cancer cells use this escape mechanism to dodge the immune system, expressing high levels of immune checkpoint molecules such as PDL1, on their surface. The interaction between PDL1 on tumor cells and its receptor PD1 on T-cells or NK cells switch these immune cells off, allowing cancer cells to escape immune surveillance. A type of immunotherapy called immune checkpoint inhibitors (PD1/PDL1 inhibitors or CTLA4 inhibitors) blocks these checkpoint molecules (PDL1) from interacting with their receptors on immune cells (PD1), allowing active T/NK cells to attack the tumor.
The gut microbiome
Who would have thought that the micro-organisms which cause us various diseases are also an integral part of our body? As soon as the field of gut-microbiota came into light, it received immense interest from researchers along with well-deserved skepticism. Although a lot of data was generated very fast, most of the studies were correlative which shows changes in microbiome in healthy vs disease conditions but fails to provide causality or functionality of these microbiomes. Development in technology has been a blessing for the field, as more and more functional studies have come up recently. Advancement in next-generation sequencing along with the use of germ-free mice or even treatment with antibiotics, researchers are able to provide functional evidence for the involvement of microbiome. One of the highlights has been the impact of gut-microbiome on cancer immune-therapy. Studies comparing the microbiota of responders and non-responders of anti-PD1 therapy show that patients who responded to the therapy had a higher diversity of intestinal microbiome compared to non-responders. The question that remained was which microorganisms are necessary for this beneficiary effect?
Isolation of IFNg+CD8+ T-cells inducing bacterial strains
On analyzing the intestinal T cells between specific pathogen-free (SPF) and germ-free mice, Tanoue and Morita et al. observed interferon-g (IFNg)-expressing CD8 T cells were enriched in the intestine of SPF mice, which decreased upon treatment with an antibiotic cocktail. On the other hand, the deficit in the germ-free mice was overcome by oral administration of faecal suspension from SPF mice. The results suggest that intestinal accumulation of IFNg+CD8+T cells is inducible and reversible.
The group then focused on the human fecal microbiota and orally administered fecal samples from six healthy humans to germ-free mice and checked the extent of intestinal IFNg+CD8+T cell induction. Thereafter, they selected the mouse with best induction of T cells (B5) after human fecal transfer and orally administered its caecal contents into fresh germ-free mice (GF-B5) along with a separate group with chloroform treated caecal contents. Chloroform treatment abrogated the T-cell induction and served as a control.
To select the stains Tanoue and Morita et al. picked 206 bacterial colonies and used 26S ribosomal RNA gene sequencing to select 26 unique strains. Five out of twenty-six strains were common with chloroform treated mice so those were neglected. Out of remaining 21 strains, 11 showed positive correlation with the frequency of IFNg+CD8T cells frequency whereas the remaining 10 didn’t show any correlation. In a time, course analysis, mice inoculated with the selected 11 strains showed increase in IFNg+CD8T within a one-week period and persisted stably for 6 months without histological or transcriptional signs of inflammation in the colon.
In further analysis of the 11 strains, no virulence factor or toxin was found and none of the strain demonstrated multidrug resistance. The phylogenetic comparison revealed 7 Bacteroidales and 4
So, what is the mechanism?
To hunt for the mechanism by which these 11-mix bacterial strains promote the induction of intestinal IFNg+CD8T cells in germ free mice, Tanoue and Morita et al. first treated the mice with heat-killed 11-mix which failed to show increase in intestinal T-cell accumulation. They further analyzed the location of the 11-mix strains in the intestine by fluorescence in situ hybridization technique and found out that the stains were able to enter the colonic mucus layer but not to the epithelium, suggesting the possible role of bacterial strains-mediated cytokine in the T cell induction.
Indeed, they observed an increased levels of chemokines Cxcl9 and Cxcl10 as well as other IFN-inducible genes in colonic epithelial cells. Furthermore, Ifngr knockout mice displayed markedly decrease induction of IFNg+CD8T cells, suggesting the possible role of IFNg-regulated gene in IFNg+CD8T cells recruitment and accumulation. On further analysis, the group noticed proliferation of IFNg+CD8T cells in the gut, marked by their ki67 expression. Implying, that cellular expansion might be partially involved in T cell-induction. The group then asked the question if the T cells were able to recognize the bacterial antigens derived from the 11 strains? They noticed an increase in IFNg+T cells when stimulated with the bacterial antigens, suggesting the bacterial antigen mediated differentiation of T cells. Furthermore, defects in dendritic cells abolished the 11-mix mediated T-cell induction. The effect was further validated to be dependent on MHC class Ia.
Protection against microbial infection
Tanoue and Morita et al. then asked if the 11-strains mediated immunity could protect against pathogenic microbial infection. Upon infection with Listeria monocytogenes, theynoticed enhanced clearance of the pathogen marked by improved colon histology score, reduced weight loss and decrease in Listeria colony-forming unit compared to germ-free mice.
Enhanced anti-cancer immunity
To check if the increased accumulation of activated IFNg+T cell after colonization with the selected 11-strains demonstrate anti-cancer property, the group used a colon cancer mouse model by subcutaneously engrafting MC38 adenocarcinoma cells to germ-free and GF+11-mix mice. Notably, GF+11-mix mice demonstrated significant anti-tumor effect which was boosted in presence of anti-PD-1 antibody compared to germ-free mice. Furthermore, depletion of CD8 T cells abolished the anti-tumor effect in 11-mix colonized mice. Suggesting that the 11-strains enhanced both spontaneous and immune checkpoint inhibitor-mediated anti-tumor effect in CD8 T cells dependent manner. In addition, the group also noticed that the combination therapy also resulted in increased GrB+IFNg+T cell and MCH class I expressing dendritic cells tumor infiltration, markers for T cell activation. Furthermore, 11 mix colonization was also effective in combination with anti-CTLA4 in MC38 model and with anti-PD-1 in BrafV600EPten-/- melanoma model.
Overall, Tanoue and Morita et al. have identified 11 bacterial strains which after colonization in mice intestine can collectively induce IFNg+T cells accumulation and activation leading to anti-microbial and anti-tumor effect. The 11-mix induced T cells further potentiated the anti-tumor effect of immune check-point inhibitors in colon and melanoma mouse models.
By Shishir Pant
Reference
Tanoue, T., et al. (2019). “A defined commensal consortium elicits CD8 T cells and anti-cancer immunity.” Nature 565(7741): 600-605.